Tech #1061: Identifying small molecule KCNB2 inhibitors as a novel targeted therapy for medulloblastoma
Medulloblastoma (MB), the most common malignant pediatric brain tumor, urgently needs targeted therapies due to severe long-term treatment effects and fatal recurrence; using a Lazy Piggy transposon screen, the Huang Lab identified KCNB2 as a key regulator of MB growth through its control of potassium flux, cell volume, membrane tension, and EGFR signaling. In SHH MB models, KCNB2 deficiency reduced tumor burden, prolonged survival, and synergized with anti-SHH therapy (vismodegib) to enhance treatment efficacy.
IP&C is seeking partnerships to complete preclinical development of the lead compounds and fund medicinal chemistry optimizations before entering IND-enabling studies.
Technology Reference Number
#1061
Inventors
IP&C Contact
Publications
Patents
To be filed following optimization of lead compounds.
Category
Therapeutics
Keywords
Medulloblastoma, SHH, SMO, pediatric brain cancer, potassium channel
Background
Medulloblastoma (MB) is the most common malignant pediatric brain tumor, originating in the cerebellum or posterior fossa. It is a highly heterogeneous disease, now classified into four main molecular subgroups: WNT, SHH (Sonic Hedgehog), Group 3, and Group 4. The current treatments for MB are surgical resection followed by craniospinal radiotherapy and cytotoxic chemotherapy, which has led to improved survival rates, especially in WNT and SHH subgroups. However, these aggressive treatments often result in severe long-term neurocognitive and developmental side effects, particularly in young children. And recurrence is universally fatal. There is an urgent need for targeted therapies as long-term effects of both disease and treatment impairs quality of life in MB survivors.
Invention Description
The Huang Lab has identified MB maintenance genes by engineering an in vivo insertional mutagenesis screen using the nested, double jumping, Sleeping Beauty (SB)/piggtBac (PB) hybrid transposon, coined Lazy Piggy.
The Lazy Piggy system first dysregulates gene expression in neoplastic cells of MB-prone mice to enhance tumorigenesis and subsequently restores dysregulated gene expression to identify tumor clones harboring transposon insertions in maintenance genes.
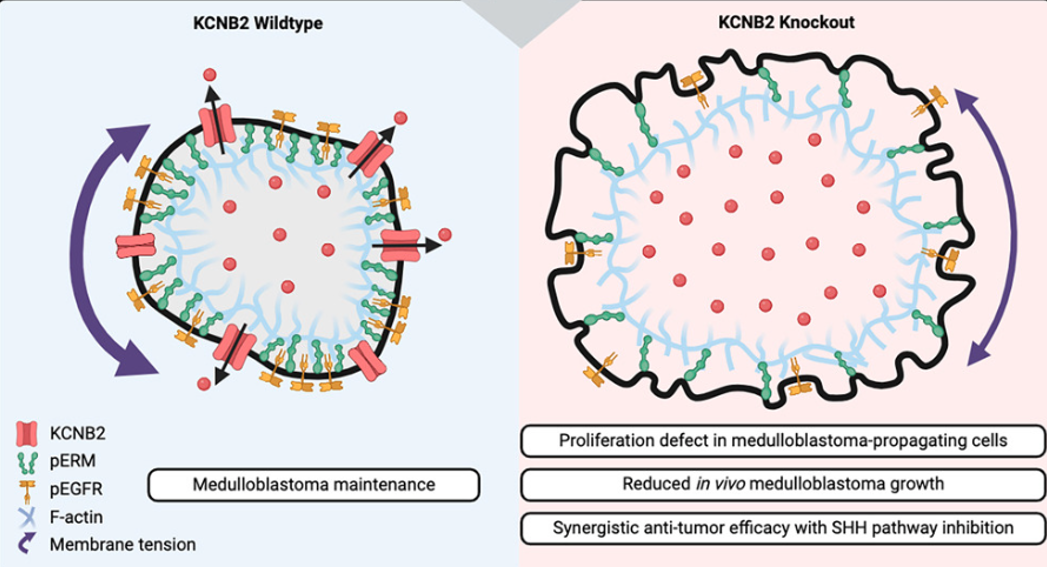
Combining Lazy Piggy screening in mice and transcriptomic profiling of human tumors discovered the voltage-gated potassium channel KCNB2. Through comprehensive in vitro and in vivo studies, the Huang Lab validated the essential role of KCNB2 and elucidated the mechanism by which KCNB2 governs MB growth (Figure 1).
KCNB2 regulates intracellular potassium levels, cell volume, and membrane tension to control epidermal growth factor receptor (EGFR) signaling and proliferation of MB-propagating cells. The absence of KCNB2 dysregulates potassium efflux, leading to osmotic cell swelling and decreased membrane tension. This decrease in membrane tension facilitates the endocytosis of transmembrane proteins, such as EGFR, reducing EGFR signaling in SHH MPCs and causing tumor growth defects (Figure 2).
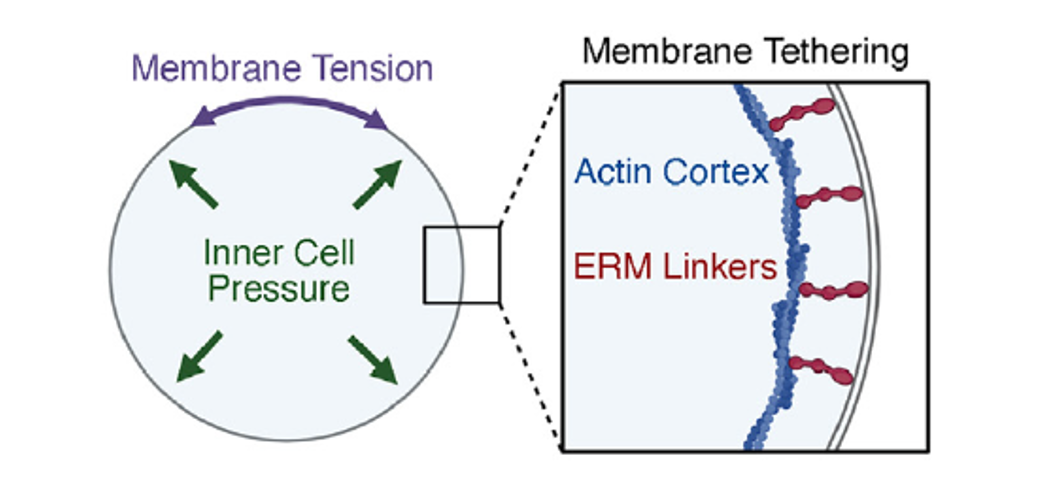
Figure 2.
Schematic illustrating concepts of membrane tension, inner cell pressure, and membrane tethering.
To investigate whether KCNB2 could serve as a drug target, the developmental phenotype of global Kcnb2 knockout mice was examined and mice are viable and produce offspring at expected Mendelian ratios. No defects in body weight at weaning, litter size, gross morphology, or brain morphology were observed.
In a preclinical model of SHH MB, KCNB2 deficiency prolongs survival and reduces the tumor burden in tumor bearing mice in an allele dose dependent manner. Specifically, it was discovered that KCNB2 knockout synergizes with anti-SHH therapy (vismodegib) to enhance anti-tumor efficacy. While vismodegib alone did not improve survival compared to vehicle control in KCNB2 wild-type mice, it significantly prolonged the survival of KCNB2-deficient mice with SHH MB. (Figure 3).
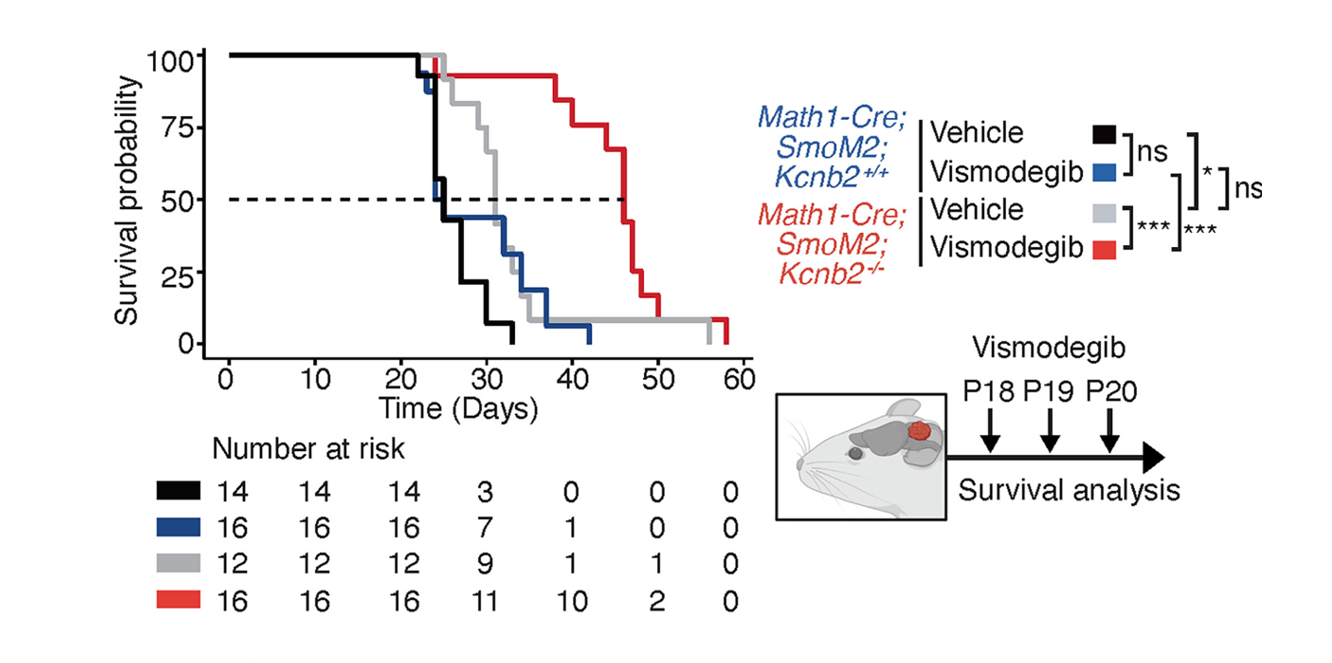
Figure 3.
Overall survival in Kcnb2+/+ versus Kcnb2−/− mice predisposed to SHH MB (left) on 3-day treatment regimen with vismodegib or vehicle (right, schematic). Pharmacologic inhibition of hedgehog pathway in SHH MB cooperates with Kcnb2 loss of function to prolong survival.
Commercial Applications
- MB represents 20% of pediatric brain tumors.
- The SHH MB sub-group accounts for 30% of all cases and occur across age groups (infants to adults).
- KCNB2 is frequently altered in other cancer types (breast, prostate, melanoma, lung and pancreatic).
- Potential for developing a combination therapy product with FDA approved SMO inhibitors like visomodegib and sonidegib that were tested in early SHH MB clinical trials with limited efficacy.
Developmental Stage
- Genetic validation of KCNB2 in a SHH MB preclinical model
- Completed 30,000 small molecule screen pre-selected for their ability to pass the blood-brain barrier
- Lead molecules have strong inhibition (>50%) in the low micromolar range and have diverse chemical structures
- Investigating the therapeutic efficacy of the ranked molecules in preclinical animal models to identify lead candidates
- Lead candidates will be optimized for potency and bioavailability