Tech #1358: A dual-action peptide for treatment of glioblastoma by targeting cancer cells and immunoactivation
Glioblastoma (GBM) is an aggressive brain cancer with poor survival outcomes, limited response to standard therapies like temozolomide, and no effective treatment for recurrence, highlighting the urgent need for targeted therapies. Dr. Xi Huang’s team developed a designer peptide that specifically targets a novel ion channel complex unique to GBM cells, killing both primary and TMZ-resistant tumors, and has the potential to enhance immunotherapy.
IP&C is seeking investments and/or a strategic partnership with a pharmaceutical company to complete development of the peptide therapeutic program.
Technology Reference Number
#1358
Inventors
IP&C Contact
Publications
Targeting network circuitry in glioma. Nature Cancer 4, 1406–1407
The never-abating excitement for targeted therapies. Nature Cancer 4, 1397-1398
Patents
Two patent families have been filed:
- National phase patent applications in the US, Canada, Europe, Australia and China covering the designer peptide’s composition of matter; methods of treating GBM: WO 2023/065046 A1.
- A PCT application covering the designer peptide’s immuno-stimulatory properties: PCT/CA2024/050514.
Category
Therapeutics
Keywords
Peptide therapy, cancer, glioblastoma, ion channel, immunotherapy
Background
Glioblastoma (GBM), the most common and aggressive brain cancer, accounts for 60% of adult brain tumours. The median survival of GBM patients is 15 months, where the standard-of-care is a combination of surgery, radiation, and chemotherapy using the DNA alkylating agent temozolomide (TMZ), which extends patient survival by only ~2 months. However, TMZ is not cancer cell specific, resulting in neural, hematological, and gastrointestinal toxicities. 50% of patients do not respond to TMZ due to the tumour’s heterogeneity. Moreover, 90% of patients develop recurrence, where there is no standardized care for recurrent GBM, and the median survival is <6 months. This highlights the demand to develop an effective, cancer-cell-specific therapy. We describe an invention that targets a non-conventional molecular target specific to GBM cells.
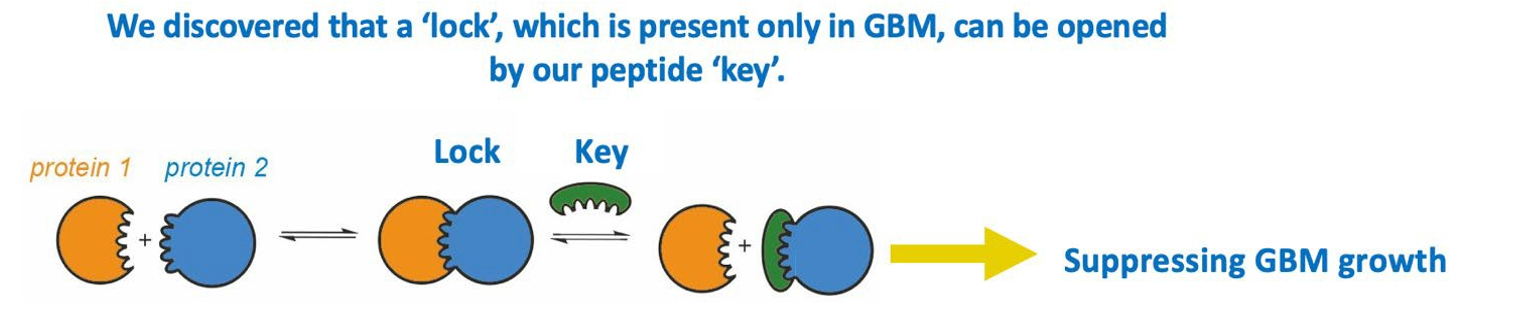
Figure 1.
We discovered a unique ion channel complex which is required for GBM to grow. We engineered a designer peptide to disrupt the assembly of this ion channel complex, thereby suppressing GBM growth.
Invention Description
Dr. Xi Huang’s team discovered a novel ion channel complex (potassium channel EAG2 and Kvβ2) expressed only in GBM cells that promotes tumour growth. Due to its absence in non-tumour cells, this interaction was targeted using a designer peptide as GBM therapy (Fig. 1). The designer peptide was administered through cannula mediated delivery, precisely killing GBM cells and improving overall survival of the mice. Using mouse models of TMZ-resistant GBM, the designer peptide showed robust efficacy in killing TMZ-resistant GBM cells and significantly improved mice survival (Fig. 2).
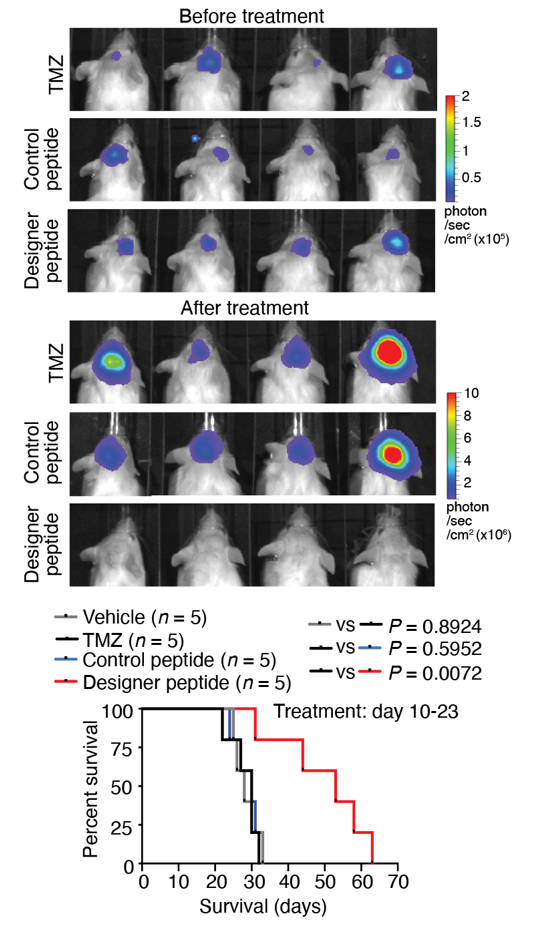
Figure 2.
Treatment with designer peptide in mice bearing patient-derived GBM tumours demonstrated our designer peptide’s robust therapeutic efficacy in mitigating TMZ-resistant GBM.
Mechanistically, the designer peptide disrupts the interaction between tumour cells and neurons, thereby mitigating GBM growth, invasion, and chemoresistance (Fig. 3).
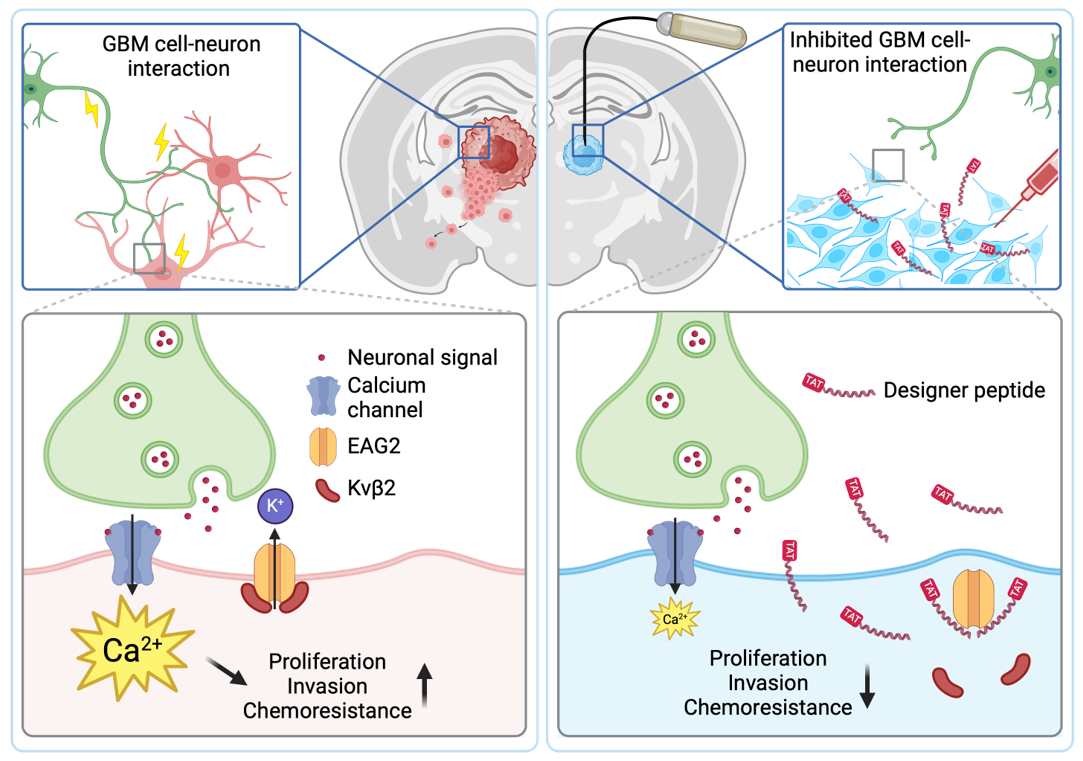
Figure 3.
EAG2 and Kvβ2 physically interact to form a potassium channel complex at neuron-GBM contact sites. EAG2-Kvβ2 complex regulates Ca2+ transients of GBM cells and promotes GBM growth, invasion, and chemoresistance. Designer peptide K90-114TAT disrupts EAG2-Kvβ2 complex formation, mitigates GBM aggression, and is efficacious in treating TMZ-sensitive and -resistance GBM.
Immunotherapy has demonstrated remarkable therapeutic efficacy in treating multiple human malignancies. However, GBM fails to respond to immunotherapy due to lack of infiltration of effector T cells, natural killer cells, and antigen-presenting cells, while harboring abundant immunosuppressive cells. Strikingly, Dr. Huang’s team discovered that the designer peptide robustly turns the “immunologically cold” GBM into “immunologically hot” tumours (Fig. 4), highlighting the potential to combine designer peptide treatment with immunotherapy to achieve long-term disease remission.
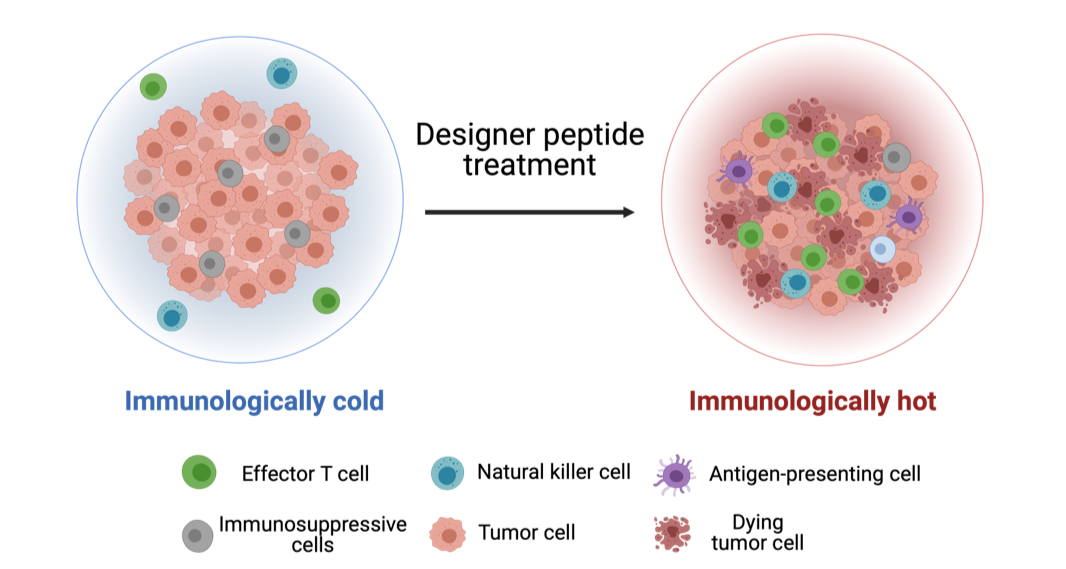
Figure 4.
Designer peptide treatment increases the infiltration of effector T cells, natural killer cells, and antigen-presenting cells while decreasing immunosuppressive cells, thereby activating the immune microenvironment of GBM.
Commercial Applications
First, as the designer peptide targets an ion channel complex that is present in tumour cells but not non-tumoural cells, it provides GBM-targeting specificity that is absent in the current standard-of-care. No side effects were detected in all preclinical mouse model studies. Second, the designer peptide treatment addresses 50% of GBM patients who do not respond to TMZ. Third, the designer peptide profoundly activates the immune microenvironment of GBM. Collectively, these findings support the potential of the designer peptide in:
- Treating TMZ-resistant GBM.
- Replacing TMZ as an effective and safe therapeutic agent for treating GBM
- Combining with immunotherapy for treating GBM.
Developmental Stage
- In vivo assessment in treating TMZ-sensitive GBM is complete.
- In vivo assessment in treating TMZ-resistant GBM is complete.
- In vivo assessment of immunoactivation of the GBM is complete.
- In vivo assessment of designer peptide treatment + immunotherapy is ongoing.
Media Coverage
SickKids News: Designer Peptide Targeting Protein-Protein Interaction Could Become Glioblastoma Treatment
Inside Precision Medicine: Cracking the cancer-neuroscience connection
Department of Molecular Genetics, University of Toronto: Researchers pave the way for new glioblastoma treatment

