Tech #1403: Small Molecule CFTR potentiator as a therapy for Chronic Obstructive Pulmonary Disease (COPD)
Chronic obstructive pulmonary disease (COPD), caused by environmental toxins like tobacco smoke, leads to airway obstruction and diminished airflow, with current treatments focusing on symptom management. Researchers from SickKids discovered a series of CFTR potentiators (SK) that were shown to prevent mucositis and could offer a potential disease-modifying therapy for COPD.
IP&C is seeking to license the technology to the pharmaceutical industry.
Technology Reference Number
#1403
Inventors
Ling-Jun Huan, The Hospital for Sick Children
IP&C Contact
Publications
Patents
Patent WO/2024/040350 Pyridazinone Compounds Which Modulate Mutant Proteins for Treating Respiratory Diseases was filled on 08/27/2023 in Canada, USA, Europe, Japan and China.
Category
Therapeutics
Keywords
Chronic obstructive pulmonary disease (COPD), CFTR potentiators, Mucositis prevention, cystic fibrosis, disease-modifying therapy, intranasal delivery
Background
Chronic obstructive pulmonary disease (COPD) is induced by environmental toxins and severely compromises breathing. It is associated with chronic bronchitis with obstruction of the large and small airways leading to diminished airflow. Currently, treatments for COPD aim to manage symptoms using bronchodilators to improve airflow and antibiotics to eradicate pulmonary infections. New disease modifying therapies are needed to treat COPD.
The CFTR chloride channel, situated on the luminal membrane of airway surface epithelium, enhances the fluidity of the airway surface liquid, thereby preventing mucus obstruction. Tobacco smoke reduces the functional expression of CFTR on the epithelial surface, exacerbating mucositis and obstruction. These findings support the hypothesis that smoke exposure induces CFTR deficiency, also known as “acquired Cystic Fibrosis” and small molecules that potentiate activity of the remaining CFTR function, will reverse the initial stages of COPD pathogenesis.
Invention Description
Researchers from SickKids discovered a series of CFTR potentiators (SK) which were tested in primary human bronchial epithelial cells from 5 lung transplant donors exposed to cigarette smoke extract. The cells were pretreated with vehicle (DMSO), Ivacaftor (VX-770), the FDA approved CFTR potentiator marketed as Ivacaftor, or SK potentiators for 24 hours. SK potentiators prevented mucositis induced by cigarette smoke extract (Fig.1).
The cultures pretreated with the SK-9919 potentiator, showed a significant increase in bead velocity – the in-vitro measure of improved hydration and mucociliary movement. The increase in bead velocity with the VX-770 was not statistically significant.
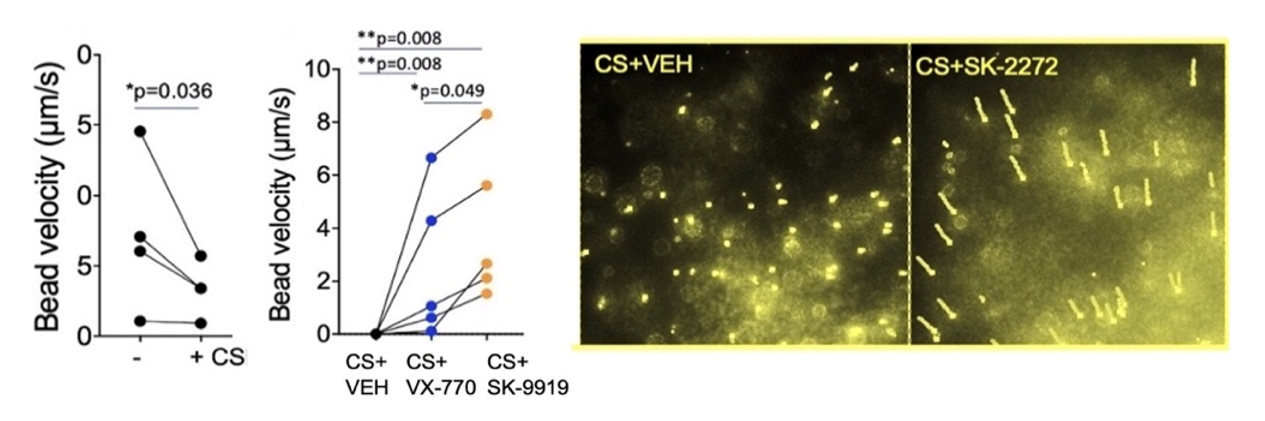
Figure 1.
Efficacy of small molecules: SK-9919 and SK-2272 in inducing mucus ciliary movement confirmed in imaging studies of primary human airway cultures
Commercial Applications
- First in class treatment of COPD
- Unique target for COPD
- Disease modifying mechanism of action
- Intranasal delivery (work in progress)
Developmental Stage
Pre-clinical

