Tech #1427: First-in-Class Anti-Toxin Therapeutic for Clostridioides difficile
Clostridioides difficile (C. diff) is a leading cause of hospital-acquired diarrhea and colitis, with recurrent infections affecting 20-30% of patients, highlighting the need for better therapies. SickKids researchers, in collaboration with the Dosa Lab, developed novel bile acid variants that remain in the gut, inhibit the potent TcdB toxin, and prevent disease recurrence in mouse models, offering a promising oral alternative to current expensive and limited therapies.
IP&C is seeking an industry partner to complete development and commercialization or investors to launch an enteric pathogen focused start-up company.
Technology Reference Number
#1427
Inventors
IP&C Contact
Publications
Patents
Filed a US provisional patent application on the novel class of compounds targeting TcdB.
Category
Therapeutics
Keywords
Clostridium difficile, Clostridioides difficile, bile acid, TcdB, gut microbiota
Background
Clostridioides difficile (C. diff), a spore forming bacteria, is the leading cause of hospital-acquired diarrhea and antibiotic associated colitis. Disruption of the protective gut microbiota by antibiotics creates a niche and enables colonization of opportunistic multidrug resistant C. diff. Once colonized, C. diff. releases the highly potent toxin TcdB that destroys the colonic epithelial barrier, resulting in clinical outcomes that range from diarrhea to life-threatening toxic megacolon. One major clinical challenge with C. diff is recurrent infection, which occurs in 20-30% of patients and >60% of patients that have had three or more C. diff infection episodes.
Previous studies have shown neutralizing TcdB alone is sufficient to prevent primary C. difficile infection (CDI) and recurrence, validating TcdB as a therapeutic target. The only TcdB-targeted therapy available on the market is an injectable monoclonal antibody known as Zinplava (bezlotoxumab). However, due to its high cost ($4,000 USD/dose), its marginal efficacy against emerging more virulent forms of TcdB, and inconvenient route of administration (intravenous infusion), bezlotoxumab has been pulled from the US market in 2025. Therefore, there remains a major unmet need and an opportunity for novel small molecule based anti-TcdB therapies with lower costs, that can be orally administered and possess a broader efficacy profile against TcdB toxin variants without disturbing the protective gut microbiota.
Invention Description
The Melnyk Lab at The Hospital for Sick Children (SickKids) discovered that certain naturally occurring bile acids bind to TcdB and prevent its ability to intoxicate cells (Tam et al., PNAS, 2020). This led to the hypothesis that bile acids could potentially be given orally to treat CDI Bile acids, however, are rapidly absorbed in the gut by bile acid transporters, and thus, do not reach the colon where TcdB acts. This makes the naturally occurring bile acids not suitable for treating CDI without a careful modification to evade the transporters.
To address this, in collaboration with the Dosa lab at the University of Minnesota, SickKids researchers synthesized novel bile acid derivatives that were designed to not be recognized by bile acid transporters, and therefore, retained in the gut. Initial testing of the lead molecule (to be optimized further) revealed that it is gut-restricted, highly potent to inhibit TcdB action in vitro, non-toxic to human cells and prevents disease recurrence in in vivo mice models (Figure 1 & 2).
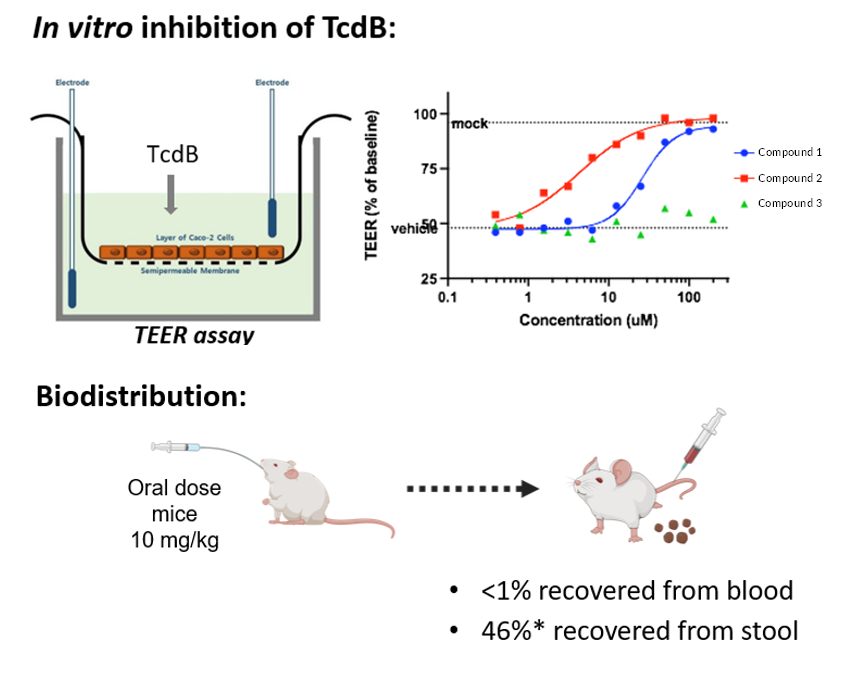
Figure 1.
Lead compounds inhibit TcdB in vitro and demonstrate gut-restriction following oral dosing.
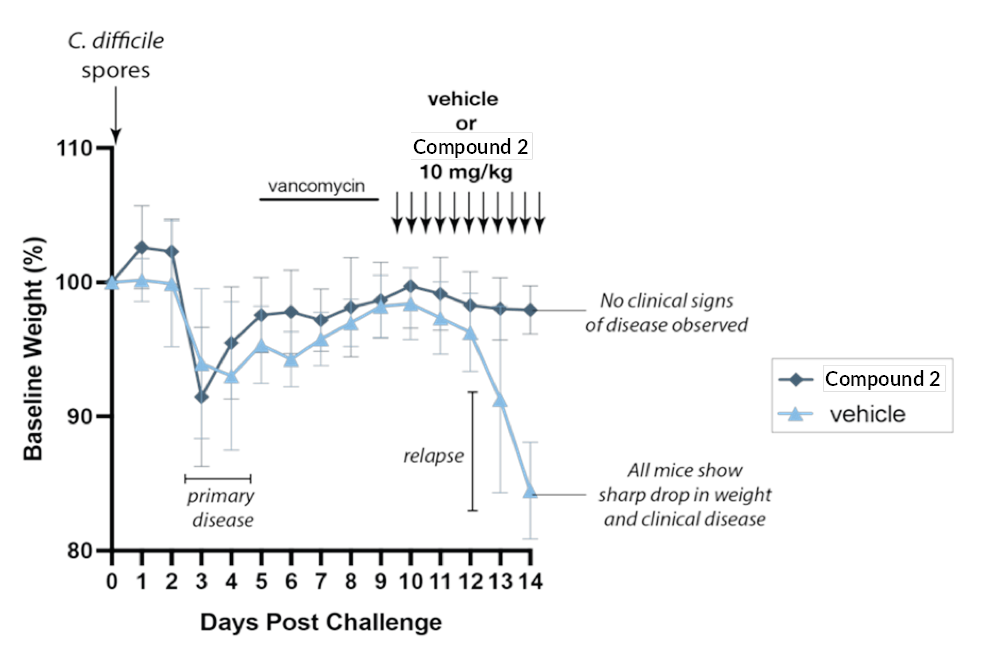
Figure 2.
Testing in C. difficile recurrence model. Mice were treated with an antibiotic to disrupt gut microbiota, then given C. difficile spores to allow disease development. Mice were then treated with vancomycin to decrease bacterial load and allow mice to recover. Upon removing vancomycin, C. difficile rapidly returns and disease relapses (light-blue curve). However, mice treated with lead bile acid variant molecule did not develop any clinical sign of disease (blue curve).
Commercial Applications
- The global difficile infection treatment market is estimated at $1.1B USD in 2022 with a projected CAGR of 6-8% (2022-2025).
- Bile acid-based therapeutics are safe and do not disrupt the natural beneficial gut microbiota.
- Prior FDA approval of Relyvrio™ (sodium phenylbutyrate/taurursodiol) for the treatment of ALS demonstrates a successful regulatory history for bile acid-based therapeutics.
- Bile acid variant-based therapeutics are a superior (potent against TcdB and its variants), cost effective (small molecule), and convenient (oral route of administration) alternative to treat C. difficile infection and prevent recurrence, compared to TcdB targeted monoclonal antibody-based bezlotoxumab or FMT.
Competitive Advantages
- First-in-class oral, gut-restricted, non-antibiotic small molecule inhibitor of TcdB
- Validated therapeutic target (via FDA approval of bezlotoxumab)
- Potential to treat primary and recurrent CDI and an adjunct therapy to existing therapies
Developmental Stage
- Proof-of-concept established with synthetic gut-restricted bile acid derivative in recurrent and primary CDI animal models
- Lead molecule synthesized and is being evaluated in primary CDI and recurrent CDI models against standard of care and bezlotoxumab
- Generating preclinical dataset evaluating ADME, dosing and toxicology

