Tech #1445: A new method for assessing the efficacy of immunotherapies in type 1 diabetes
Current methods to predict type 1 diabetes (T1D) onset or response to immunotherapy lack precision, prompting the need for better diagnostic tools. The Danska Lab has developed a novel approach using antibody responses to specific gut bacteria to identify individuals likely to respond to anti-CD3 therapy and delay T1D onset.
IP&C is seeking a collaboration to develop and commercialize the new diagnostics platform.
Technology Reference Number
#1445
Inventors
IP&C Contact
Publications
Patents
PCT application Methods for stratifying subjects with type 1 diabetes for treatment with an anti-cd3 antibody and for predicting progression to type 1 diabetes #PCT/CA2024/051396 filed on 10-24-2024
Category
Diagnostics
Keywords
Type 1 Diabetes, Anti-CD3 Therapy, Gut Microbiota, Biomarkers, Immunotherapy Response
Background
Emerging immunotherapy treatments such as anti-CD3+ antibodies (Abs) to prevent type 1 diabetes (T1D) require novel diagnostic methods to identify immunotherapy responders and to predict disease onset in pre-diabetic patients. For the past 3 decades HLA-DR haplotypes and serum islet autoantibodies have been used to calculate T1D risk but have limited precision to predict time to diagnosis, or response to therapy.
Invention Description
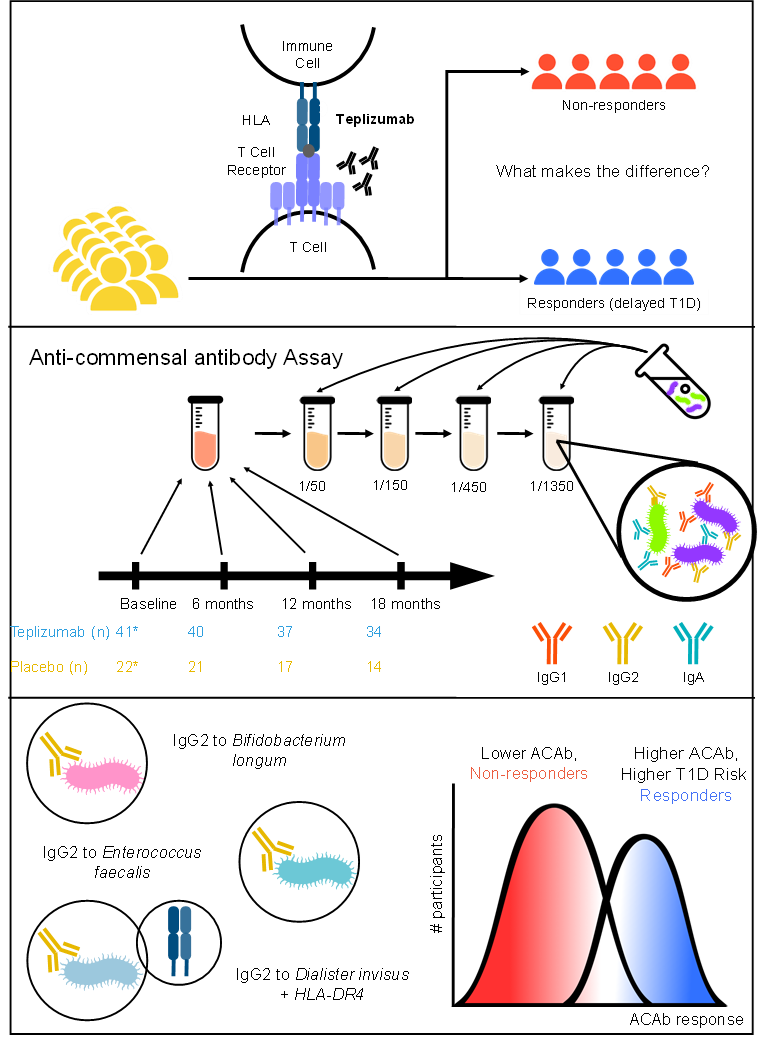
The Danska lab has developed a new method to identify responders/non-responders to anti-CD3 Abs in T1D at-risk individuals. They assessed Ab responses against a panel of taxonomically diverse intestinal “commensal” bacteria species (anti-commensal Ab; [ACAb]) in serum from clinical study participants treated with anti-CD3 Mab teplizumab (TzieldTM) vs placebo. IgG2 responses to 3 commensal species associated with time to T1D diagnosis and Tzield responses that delayed T1D onset.
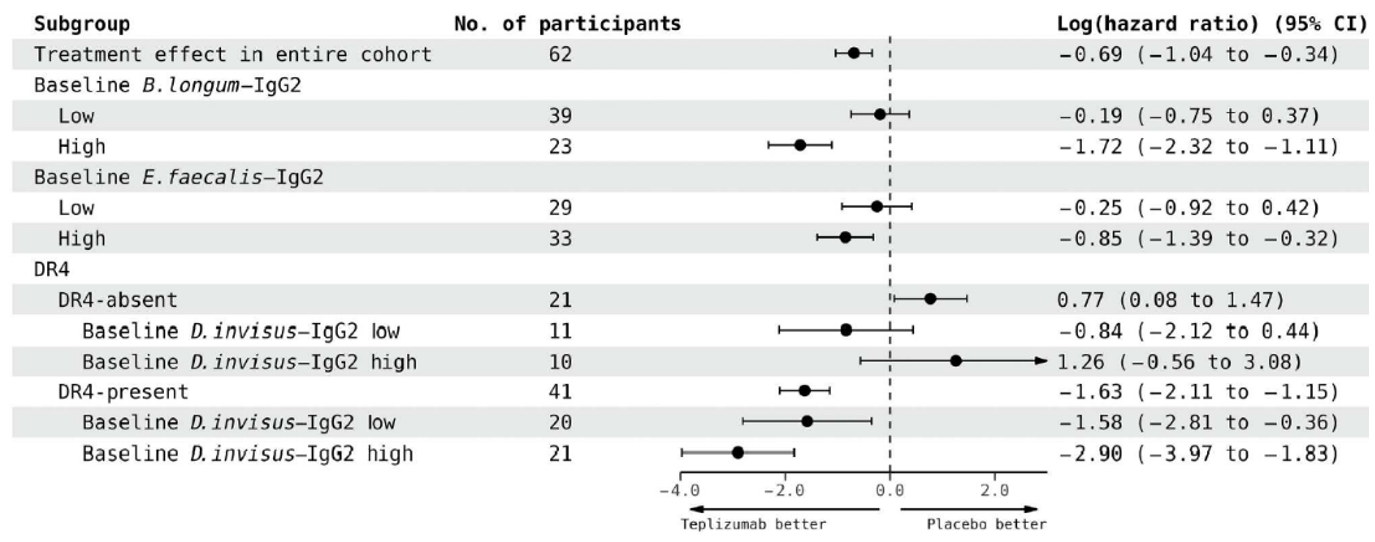
Figure 1.
Responses to Tzield are associated with IgG2 responses to selected gut bacteria before treatment.
Commercial Applications
These Ab responses are the first biomarkers linking human intestinal bacteria with T1D progression. ACAb analysis provides a new approach to identify pre-T1D (stage 2) or new onset (stage 3) T1D patients who may benefit from Tzield, now approved by the U.S. FDA for delaying TD1 onset, or emerging T cell modulators.
Developmental Stage
Granted claims are broad on the use of the salicylanilide class of compounds including niclosamide ethanolamine salt to treat (stand alone, or in combination with antibiotics as 1L, 2L) a host infected with or at risk of infection with a pathogen including C. difficile bacteria.
The granted claims are based on SickKids researchers’ original invention describing the mechanism of action of niclosamide to modify the host cells, inhibit endocytosis of C. difficile toxins, and hence prevent infection. This IP establishes the foundation for using the salicylanilide class of compounds (e.g., niclosamide) to treat and prevent infection from a broad class of pathogens.