Tech #933: Lung-specific macrophage cell therapy for lung diseases
Current lung disease treatments only alleviate symptoms without addressing the underlying dysfunctional immune response, leading to a high health and economic burden, with millions suffering from obstructive and restrictive lung diseases. The Post lab has developed a patent-protected method to differentiate human stem cells into ALMs, which can be delivered directly to the lungs to modulate the immune environment and potentially treat multiple lung diseases simultaneously.
Seeking pre-seed investment or partnership to accelerate human ALM pre-clinical studies
Technology Reference Number
#933
Inventors
IP&C Contact
Publications
Patents
Issued patents in US (11/001,806, 17/316,060), CA (2,975,780), and EP (3256569)
Pending applications in US (17/598,593), CA (9 3,134,782), EP (20776458.0), PCT (WO2024152111A1)
Category
Therapeutics – Cell Therapy
Keywords
Cell therapy, macrophage, lung disease, chronic obstructive pulmonary disease, lung cancer, NSCLC, SCLC, idiopathic pulmonary fibrosis
Background
Current therapeutics treat only symptoms of lung disease and are unable to address the underlying drivers of lung disease. Lung diseases are complicated, multifactorial, and result from a dysregulated innate immune response. Nothing currently on the market addresses that dysfunctional immune response
Millions suffer from progressive lung diseases annually, and these are broadly categorized as obstructive (COPD, asthma) or restrictive (interstitial lung disease). Patients receive limited supportive care (immunomodulatory drugs, bronchodilators, antibiotics), leading to reduced quality of life and early mortality. The US bears an economic burden of progressive lung diseases exceeding $70 billion annually.
Invention Description
The Post lab has developed a patent protected in vitro differentiation protocol whereby human stem are differentiated into hALMs in xeno-free (seeder/feeder independent) media. ALMs can be delivered directly to the lungs via intratracheal/ intrabronchial administration or, in the future, with cutting edge aerosolized delivery modalities which are currently under development.
ALMs can therefore be designed to exert a specific therapeutic effect and, given AMs central role in the immune system, can further modulate the immune environment for therapeutic gain. While ALMs may be tuned to a specific lung disease (i.e. COPD) they would also remain in the lungs and be able to respond to many common lung comorbidities like lung infections.
Some examples of ALM products tuned to specific lung diseases are: (1) unmodified ALMs can clear bacteria and prevent lung damage in rat models of PAO1 infection and mouse models of RSV infection (Figure 1), (2) IL-10 and a1 anti-trypsin secreting ALMs restore lung elasticity and function in a murine emphysema model (Figure 2), and (3) SIRPa mutant ALMs localize to and phagocytose tumor cells in rat lung cancer model (Figure 3).
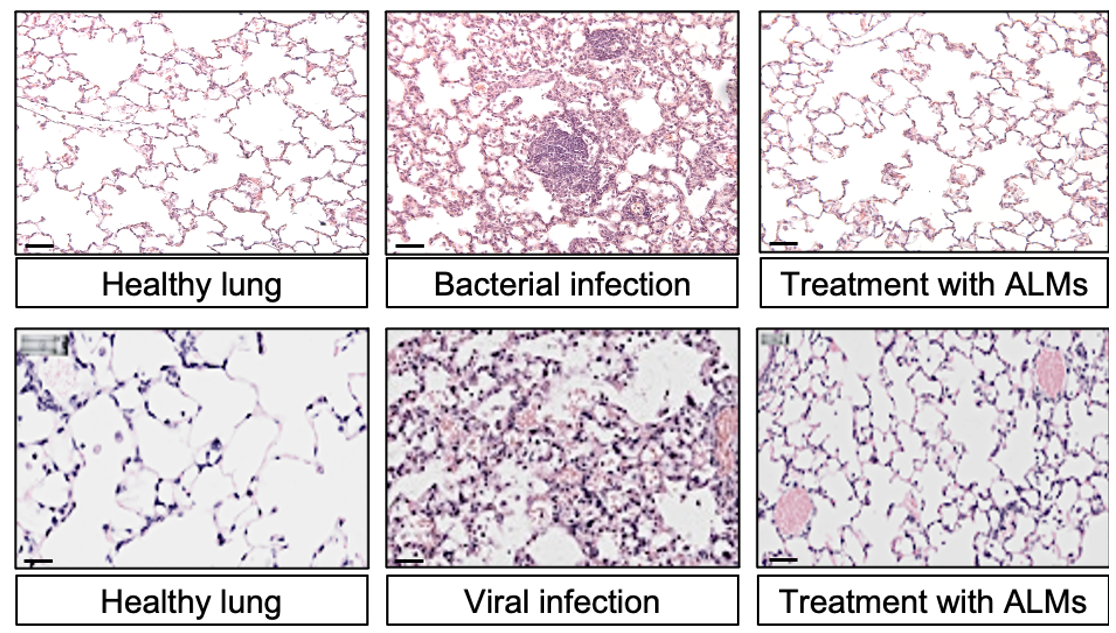
Figure 1.
Top infection with P. aeruginosa, bottom infection with RSV. Co-administration shows reduced inflammation and less infiltration with immune cells.
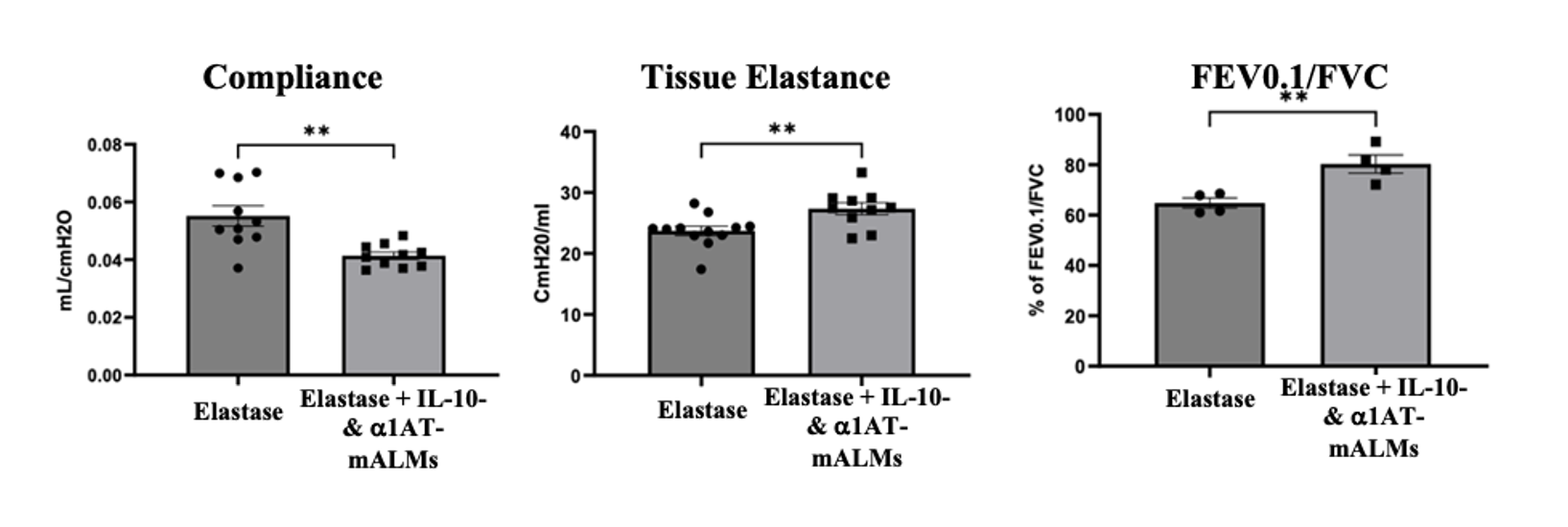
Figure 2.
Installation of ALMs secreting IL10 and a1 anti-trypsin improves lung elasticity, compliance and function.
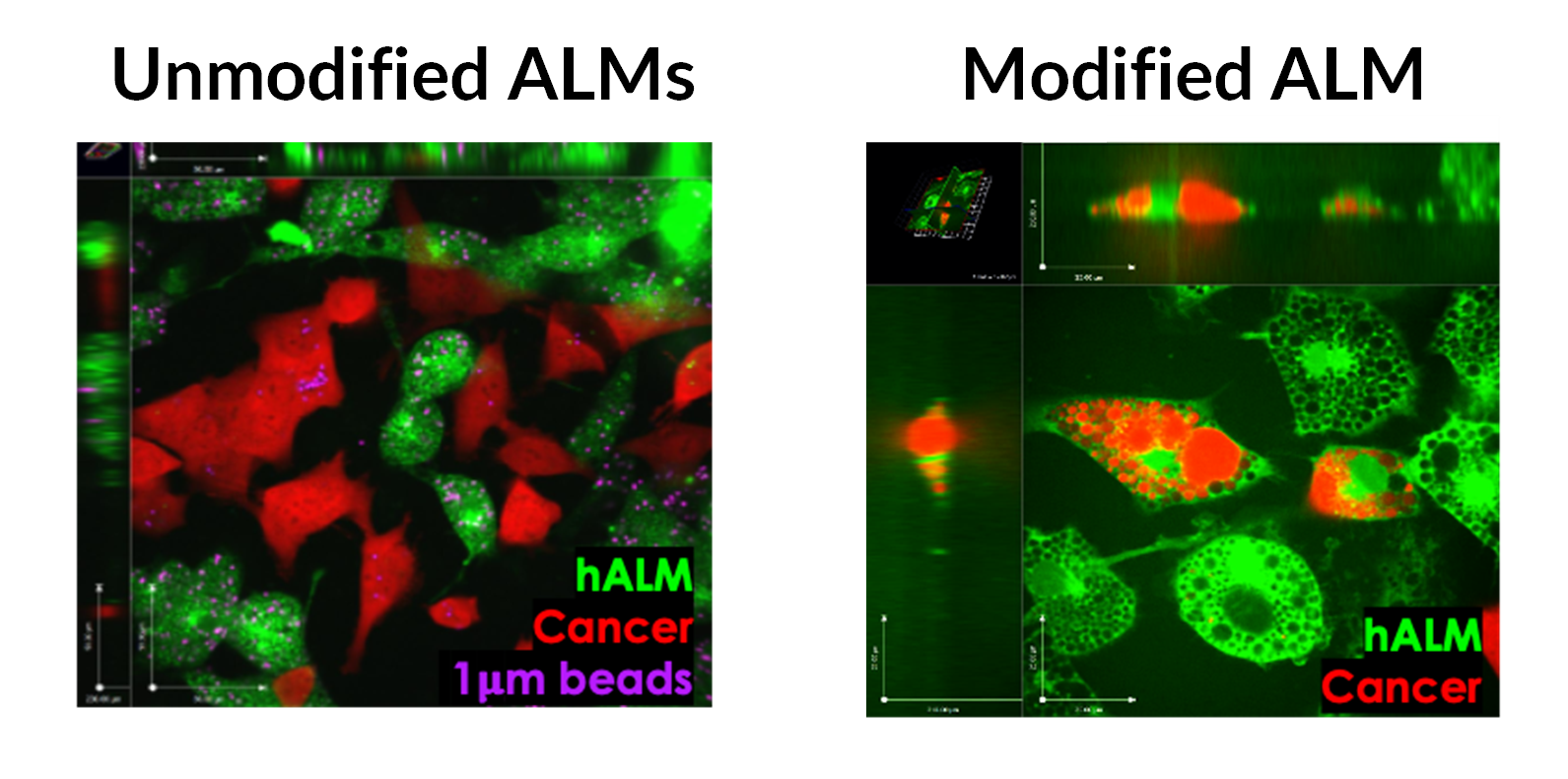
Figure 3.
Left: unmodified ALMs are unable to recognize and phagocytose cancer cells. Right: overexpression of truncated SIRPa enabled macrophages to hone to and phagocytose cancer cells in vitro.
Commercial Applications
The positioning of AM/ALM at the interface of environmental exposure, epithelial injury, inflammation, cellular remodeling/repair, and the adaptive immune system make this technology an ideal candidate to treat lung diseases and conditions that are recalcitrant to current standard of care therapies.
We see potential applications of ALMs in:
- First line treatment for fibrotic (IPF) and obstructive (COPD) lung diseases
- Lung cancer as an option for unresectable tumors or SCLC
- Infectious disease (especially recurrent/chronic and antimicrobial resistant infections, cystic fibrosis)
Developmental Stage
ALMs have been extensively characterized for cell surface markers, phagocytic activity, secretion of cytokines, and by these phenotypic measures behave as lung AMs are expected to. We have secured access to a GMP compliant iPSC cell lines with immune cloaking and favorable safety features. The team is currently generating a data package around the dose/schedule, biodistribution, PK/PD, toxicity on the human iPSC derived cells.

