Tech #1321: Clinically validated CSF-based liquid biopsy for CNS cancers
This clinically validated CSF liquid biopsy provides a fast, minimally invasive way to generate comprehensive molecular profiles for CNS tumors, overcoming the limitations of traditional surgical biopsies. By combining low-pass whole genome sequencing with a targeted DNA panel, it delivers actionable insights to guide diagnosis, treatment, and monitoring, offering a major advancement in precision oncology.
We are looking for global licensing opportunities for the CSF base liquid biopsy test
Technology Reference Number
#1321
Inventors
IP&C Contact
Patents
MSI analytic assessment: applications pending in Canada (3,200,667) and the US (18/313,101).
Category
Diagnostics
Keywords
Liquid Biopsy, blood, cerebrospinal fluid, circulating tumour cells, brain tumours, next generation sequencing
Background
Molecular characterization is essential for diagnosing cancer, guiding treatment decisions, and monitoring disease progression. However, traditional tissue biopsies are not possible for all types of central nervous system (CNS) tumors. These are often highly invasive procedures and not always physically possible if the tumour is located in the midline or deep brain regions, and given this invasive nature repeat sampling of CNS tumours is not feasible. Additionally, CNS tumours are often heterogeneous, meaning a single biopsy may not capture the full molecular landscape of the disease. These patients do not have access to non-invasive diagnostics which often leads to missed opportunities for early intervention and inappropriate or ineffective treatment choices.
Invention Description
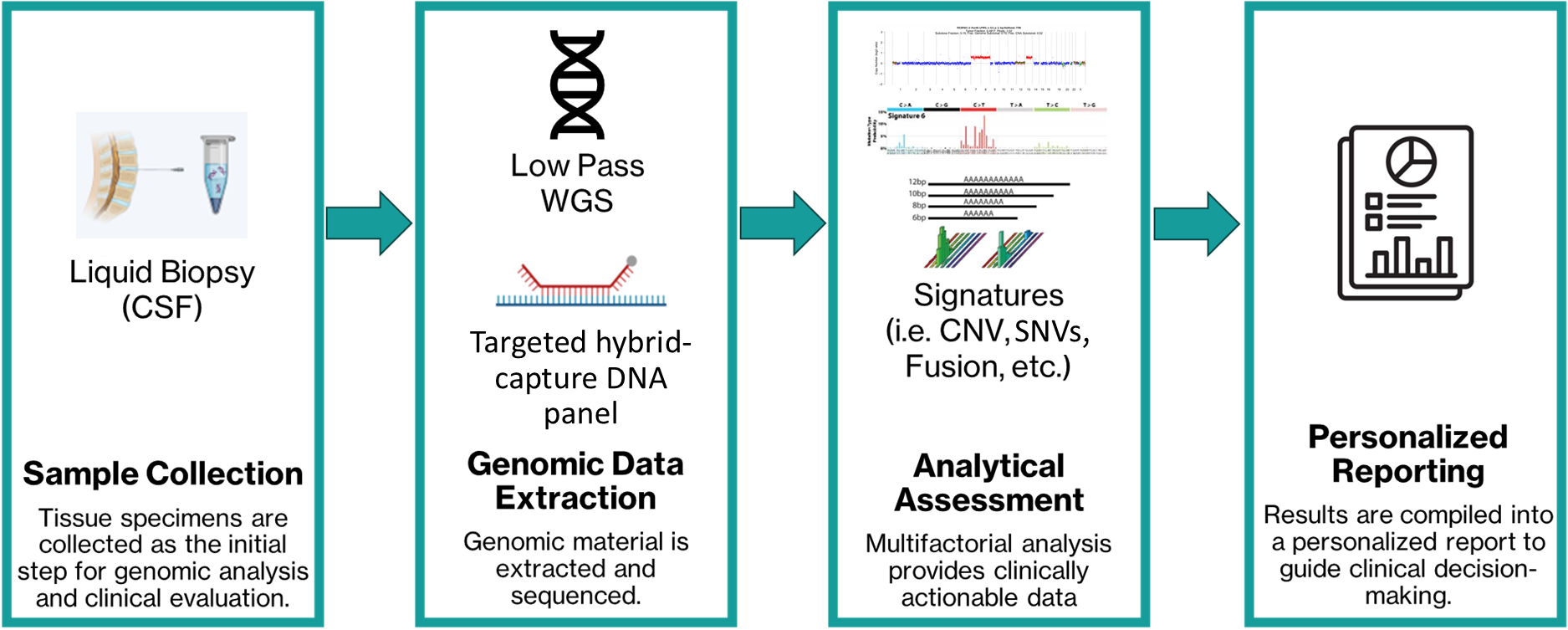
Liquid biopsy offers a minimally invasive alternative by analyzing circulating tumor DNA (ctDNA) from cerebrospinal fluid (CSF) collected from patients with CNS tumors. SickKids researchers have developed a diagnostic tool that, from a single sample of patient CSF, leverages low-pass whole genome sequencing (WGS) and a targeted DNA panel to provide robust, clinically actionable insights, into tumour biology, enabling precision medicine at scale (Figure 1).
Key Clinical Applications
Non-invasive Diagnosis: Enables molecular characterization of tumors from CSF liquid biopsies, reducing the need for risky surgical biopsies—especially critical for brain tumors and other hard-to-access cancers.
Therapy Selection & Monitoring: Identifies actionable mutations and tracks tumor evolution, residual disease, and therapy response over time, supporting dynamic treatment decisions.
Risk Stratification & Surveillance: Detects cancer predisposition syndromes (e.g., Lynch, CMMRD), guiding surveillance for patients and families.
Broad Tumor Coverage: Validated across high-grade and low-grade gliomas, medulloblastoma, germ cell tumors, and more, with high sensitivity and specificity. Can be used to characterize tumorous and follow treatment plans for both pediatric and AYA patients with cancers.
High Versatility: The test can be adapted to additional sample sources including blood and solid tissue. Additionally, the test is also useful for analyzing other solid and heme tumours.
Market Potential
- The Global market for Genetic Testing Services: Oncology Molecular Tests was $2.5B USD in 2024 and expected to grow to $3.3B USD by 20291.
- Growth in the oncology diagnostic market is driven by adoption of novel molecular tests.
- There are 3,300 newly diagnosed brain cancer patients annually in Canada (~320,0002 globally).
- CNS cancer patients undergo multiple lumbar punctures over the course of treatment. Beginning at diagnosis and continuing over the course of treatment, following minimal residual disease, response to treatment and/or tumour resistance to therapy a patient may experience 10 lumbar puncture procedures, leading to many opportunities for analysis of CSF samples.
Summary
This is a disruptive diagnostic platform offering clinically actionable information about CNS cancers from patients’ CSF samples. The combination of low-pass genome-wide analysis and a targeted DNA panel delivers fast, affordable, and comprehensive molecular profiling for CNS cancer patients. Its ability to guide therapy and enable population-scale screening makes it a game-changer for cancer care as well as a compelling market opportunity. This next generation sequencing strategy has been clinically validated and implemented at The Hospital for Sick Children, and we are looking for opportunities to increase the clinical uptake in more hospitals across the rest of the world.
References
1Worldwide Clinical Laboratory Services, 10th Edition. Kalorama Information