Tech #1343: ImmunoTargeting Vaccine Platform
The ImmunoTargeting Vaccine (ITV) is a modular, adjuvant-free subunit vaccine platform leveraging a fully humanized monoclonal antibody (44H10) targeting HLA-DR to enhance immune responses. Designed for rapid, cost-effective vaccine development, ITV addresses key challenges in global immunization by enabling strong B-cell activation, broad strain coverage, and improved thermostability for easier distribution, particularly in low-resource settings.
Seeking strategic partnership and licensing to develop immunotargeted products
Technology Reference Number
#1343
Inventors
IP&C Contact
Publications
Patents
Pending patent applications: CA 3250551 and US 18/863143
Category
Therapeutics – Vaccine Platform
Keywords
Vaccine, infectious disease, multivalent vaccine, immune stimulation, antibody, vaccine platform
Background
Our interconnected world enables rapid spread of emerging infectious diseases and variants, highlighting the need for efficient, adaptable vaccine development platforms.
Despite vaccine successes, the recent pandemic has revealed gaps and challenges, including the need for: 1) Rapid and less costly vaccine development, 2) Modular platforms with broad neutralizing capabilities against the newest strains and diseases, 3) Stronger B cell responses to prevent initial infection, and 4) Vaccines that don’t require extreme cold chain storage.
Invention Description
Jean-Philippe Julien and Brian Barber created a vaccine platform from a fully humanized monoclonal antibody recognizing HLA-DR, called the ImmunoTargeting Vaccine (ITV) (Figure 1). The platform is a modular, adjuvant-free, pan-HLA-DR-immunotargeting subunit vaccine with broad HLA-DR reactivity for effectiveness across diverse human populations (Figure 2). The ITV was created with the challenges above in mind:
- The adjuvant-free formulation bypasses complex, timely, and expensive processes of developing a new adjuvant, or testing new antigens with established adjuvants. With platform vaccines, the issue is amplified, as the adjuvant must be tested with each antigen alone, and then with the full combination of antigens.
- The antibody can be fused to up to four different cargo, including antigens and t-cell stimulatory peptides. The modular nature of the platform allows for multivalency and could address antigenic drift.
- The pan-HLA-DR monoclonal antibody (44H10) delivers viral antigens directly to antigen-presenting cells, eliciting antigen uptake and strong B-cell responses. The additional incorporation of T-cell epitopes facilitates a broader, longer-lasting immune response.
- The thermostability simplifies storage and distribution, suitable for rural locations and LMICs.
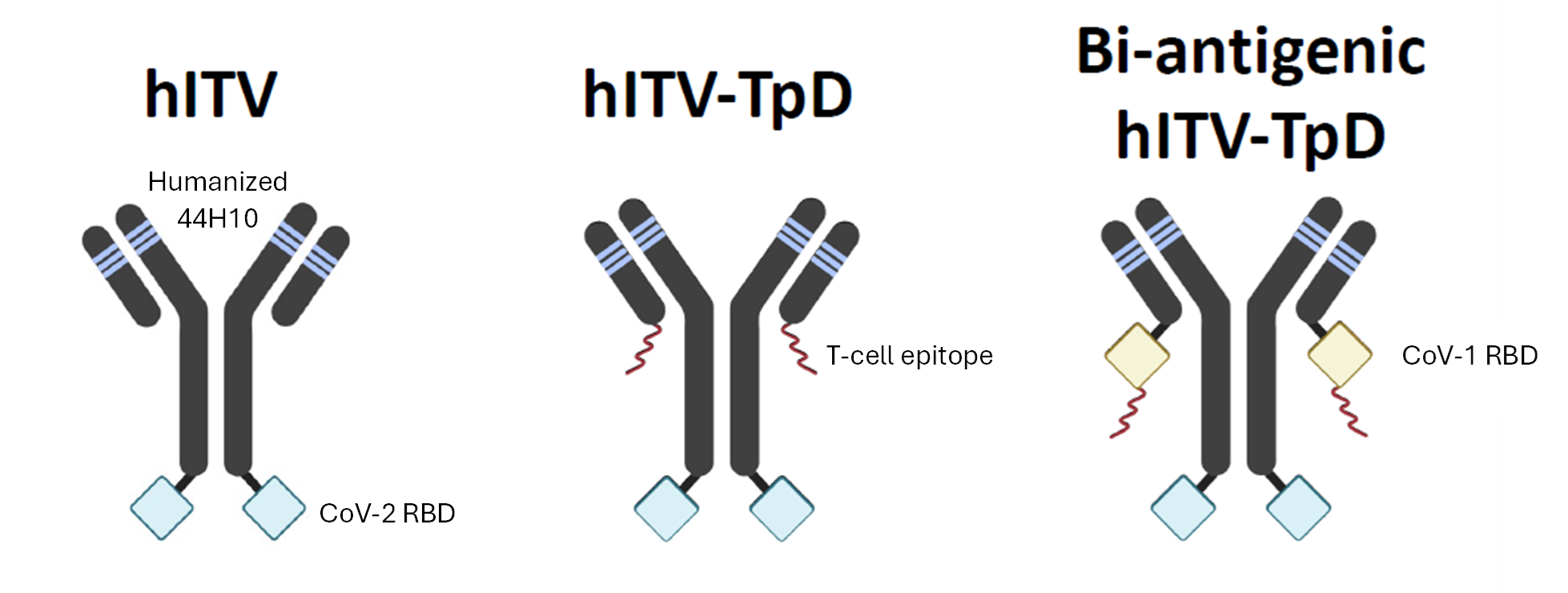
Figure 1.
Schematic of the immunotargeting vaccine (ITV). The fully humanized monoclonal antibody provides a modular, adjuvant-free, pan-HLA-DR immunotargeting subunit vaccine platform.
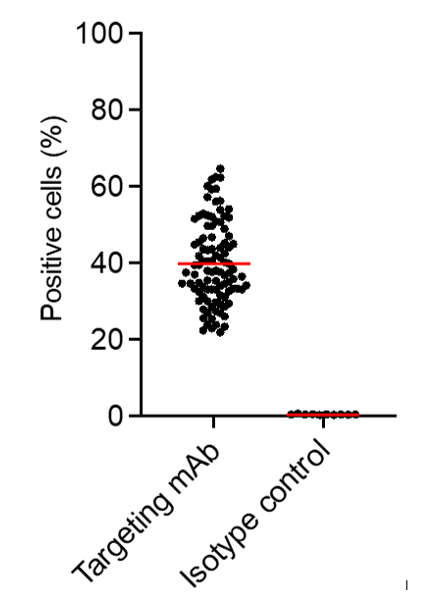
Figure 2.
Broad specificity of ITV. Data showing ITV binding to 100% of blood donor derived PBMC of 100 random samples from the Canadian Blood Services.
Commercial Applications
We believe ITV could address vaccine shortcomings and improve immune responses, especially in the most vulnerable (elderly, immunocompromised, cancer patients, non-responders), who often have weaker and rapidly waning antibody protection. It also offers pandemic preparedness by quickly engineering multiple antigens onto the platform, for broad, multivalent immune responses, with potential as both a primary vaccine series and a booster for infectious diseases.
| Vaccine Market Segment | Global Market Size (2024 – Millions, USD) |
|---|---|
| Recombinant Vaccine Market | $21,130.97 Grand View Research, Vaccine Market Outlook Report |
| COVID-19 Vaccine Market | $3,800 BCC PHM014K |
| Accessible Market | $38 1% of COVID-19 Vaccine Market |
Developmental Stage
ITV engineered to carry the RBD domain of both SARS-CoV-2 and SARS-CoV-1 has demonstrated pre-clinical POC both in in vitro PsV assays and in an in vitro rabbit model of infection (Figures 3 and 4).
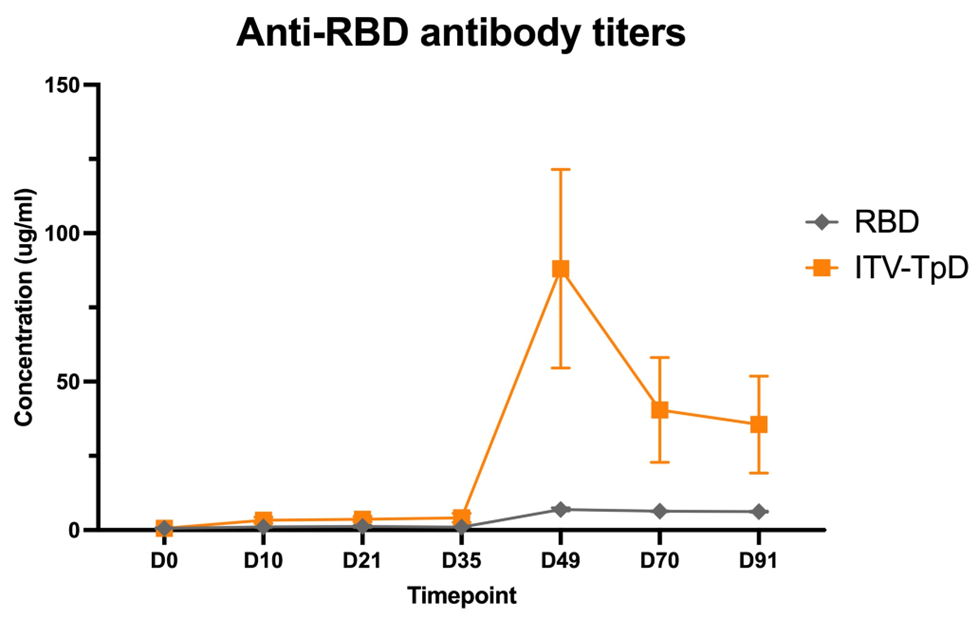
Figure 3.
Comparison of unadjuvanted RBD and ITV-TpD demonstrating the boost in antibody response for RBD on the ITV platform

Figure 4.
Demonstration of bi-antigenic ITV-TpD to elicit a strong multi-valent antibody response to both SARS-CoV-1 and SARS-CoV-2 RBD in the absence of adjuvant.
The platform is well-evaluated in adjuvant free settings and is ready for more intensive studies evaluating the potential to boost the immune response in the vulnerable.

