Tech #1343: Broad specificity HLA-DR monoclonal antibody
Aberrant HLA-DR expression contributes to hematologic malignancies, autoimmune diseases, and transplant rejection, but therapeutic targeting has been limited by polymorphism and immune risk. To overcome this, Drs. Brian Barber and Jean-Philippe Julien developed HITmAb, a fully humanized monoclonal antibody with broad HLA-DR reactivity and modular design for targeted delivery of therapeutic payloads across diverse species and human populations.
Seeking strategic partnership/licensing to develop immunotargeted therapeutic products
Technology Reference Number
#1343
Inventors
IP&C Contact
Publications
Patents
Patents pending in Canada (CA 3250551) and the US (US 18/863143).
Category
Therapeutics – Immunotargeting Monoclonal Antibody
Keywords
Monoclonal antibody, vaccine, antibody drug conjugate, immune dampening, graft vs host disease, autoimmune disease
Background
HLA-DR plays a central role in antigen presentation and immune regulation. It is expressed on professional antigen presenting cells (APCs) and is upregulated during immune activation. Aberrant HLA-DR expression is implicated in hematologic malignancies, autoimmune diseases, and transplant rejection. Despite its importance, therapeutic targeting of HLA-DR has been limited due to polymorphism and concerns about immune dysregulation.
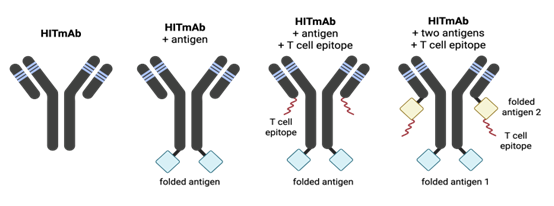
Our team aims to address these challenges with a novel antibody product exhibiting broad HLA-DR reactivity with a modular architecture (Fig. 1) allowing the fusion to antigens, epitopes, or payloads for diverse therapeutic applications.
Invention Description
Drs. Brian Barber and Jean-Philippe Julien have engineered a fully humanized Immune Targeting monoclonal antibody (HITmAb). Derived from murine antibody 44H10 and engineered using CDR grafting onto an IgG1 framework, HITmAb binds HLA-DR with nanomolar affinity and maintains high stability and specificity post-humanization. The antibody demonstrates broad allele reactivity (Fig. 2) enabling HLA-DR targeted delivery of antigens, cytotoxic agents, or immunomodulators to APCs in all humans. Additionally, HITmAb has cross-species compatibility (demonstrated in humans, rabbits, ferrets, macaques), and exhibits robust performance as an adjuvant-free vaccine in pre-clinical models (Fig. 3).
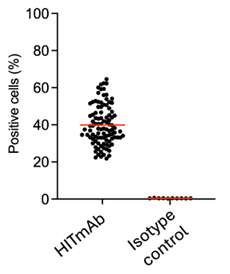
Figure 2.
Broad specificity of HITmAb. Data showing HITmAb binding to 100% of blood donor derived PBMCs of 100 random samples from a diverse population.
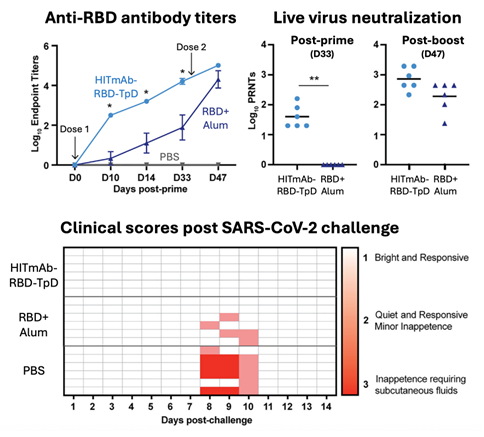
Figure 3.
Efficacy of HITmAb fused to the SARS-CoV-2 spike protein RBD and the T cell epitope TpD as a vaccine candidate in a pre-clinical ferret infection model of COVID-19. The HITmAb-RBD-TpD construct elicits robust antigen-specific antibody responses that neutralize live SARS-CoV-2 virus both before (D33) and after (D47) boosting, and protect immunized rabbits from clinical COVID-19, surpassing responses elicited by RBD adjuvanted in Alum.
Commercial Applications
In addition to infectious disease vaccine applications the platform can be adapted for:
- the delivery of tumour neoantigens to APCs thereby focusing the immune system on the recognition of these specific neoantigen-expressing tumour cells.
- the delivery of self-antigens to APCs to re-establish tolerance in the case of autoimmune diseases.
- the delivery of immunomodulatory drugs (i.e. IL-10 or TGF-b) to APCs to modulate/regulate an ongoing immune response.
- the development of novel antibody drug conjugates (ADC) that deliver toxic payloads to HLA-DR positive malignant cells.
- depleting of donor graft APCs prior to host engraftment, reducing the potential for graft vs host disease (GVHD).