Tech #1343: Clinical immunodiagnostic – immune cell specific monoclonal antibody
The high polymorphism of HLA-DR has limited the development of antibodies for use in manufacturing, histology, and flow cytometry. Drs. Brian Barber and Jean-Philippe Julien have developed HITmAb, a fully humanized monoclonal antibody with broad HLA-DR reactivity and modular architecture that enables versatile reagent applications without the need for MHC typing.
Seeking strategic partnership/licensing to develop reagent products specifically recognizing HLA-DR expressing immune cells
Technology Reference Number
#1343
Inventors
IP&C Contact
Publications
Patents
Patents pending in Canada (CA 3250551) and the US (US 18/863143).
Category
Therapeutics – Immunotargeting Monoclonal Antibody
Keywords
Monoclonal antibody, HLA-DR specific, flow cytometry, cell enrichment, ELISA
Background
HLA-DR plays a central role in antigen presentation and immune regulation. It is expressed on professional antigen presenting cells (APCs) and is upregulated during immune activation. The high degree of polymorphism in the HLA-DR gene has prevented the use of antibodies to this important receptor in manufacturing, histology, and flow-cytometry.
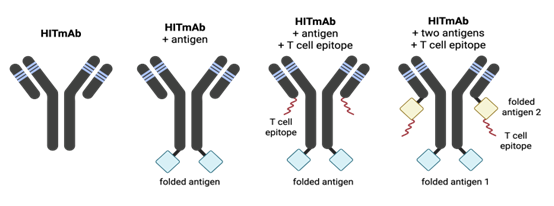
Our team aims to address these challenges with a novel antibody product exhibiting broad HLA-DR reactivity with a modular architecture (Fig. 1) allowing the fusion to nanoparticles, detection agents like fluorophores, or surfaces for diverse reagent-based applications.
Invention Description
Drs. Brian Barber and Jean-Philippe Julien have engineered a fully humanized immune targeting monoclonal antibody (HITmAb). Derived from murine antibody 44H10 and engineered using CDR grafting onto an IgG1 framework, HITmAb binds HLA-DR with nanomolar affinity and maintains high stability and specificity post-humanization. The antibody demonstrates broad allele reactivity (Fig. 2) essentially reacting with HLA-DR in all humans, thereby eliminating the need for MHC typing when used with individual humans. Additionally, HITmAb has cross-species compatibility (demonstrated in humans, rabbits, ferrets, macaques), and exhibits exceptional stability and favorable manufacturing characteristics (Fig. 3).
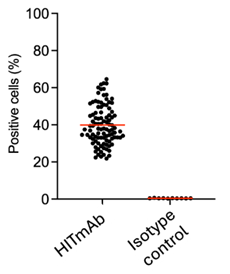
Figure 2.
Broad specificity of HITmAb. Data showing HITmAb binding to 100% of blood donor derived PBMCs of 100 random samples from a diverse population.
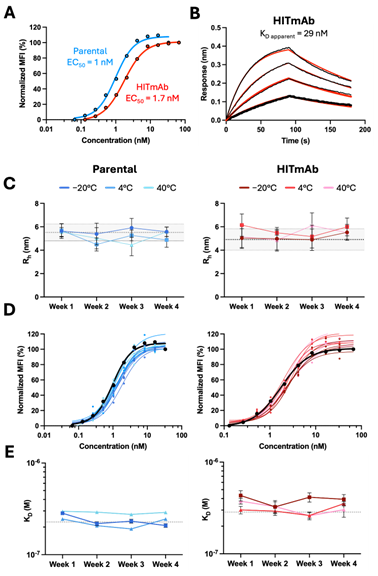
Figure 3.
HITmAb displays biophysical properties and thermostability similar to the originating mouse parental antibody. (A) Flow cytometry titration curves comparing parental and HITmAb binding to BJAB cells. (B) BLI binding of HITmAb to HLA-DR, where black lines represent measured binding and red curves correspond to the data fitted to a 1:1 binding model. (C–E) Biophysical characterization of parental and HITmAb kept at various storage temperatures for up to four weeks, including hydrodynamic radii and polydispersity measured by DLS (C), binding to BJAB cells measured by flow cytometry (D) and binding to recombinant HLA-DR measured by BLI (E).
Commercial Applications
Potential applications for a reagent HITmAb include:
- Sorting for HLA-DR positive cells in blood flow cytometry
- As a reagent in immunobiological analysis of human tumour tissues to identify the presence of HLA-DR positive APCs
- As a manufacturing reagent to remove HLA-DR positive cells from a bulk mixture (e.g. stem cell transplantation applications)