LATEST PUBLICATION
Targeted Anti-Toxin Therapeutic Shows Major Promise Against C. difficile in SickKids-Led Study
Clostridioides difficile (C. diff) is a leading cause of hospital-acquired infections, responsible for severe gastrointestinal illness and high rates of recurrence. Despite the availability of antibiotics, many patients enter a cycle of repeated illness due to disruption of the gut microbiome and re-exposure to toxin-producing bacteria. To break this cycle, new therapeutic strategies that target the root drivers of disease are urgently needed.
Researchers at The Hospital for Sick Children (SickKids), have uncovered how naturally occurring bile acids bind to and inhibit C. diff’s most potent virulence factor, toxin B (TcdB). Their findings, published in Nature Microbiology, enabled the design of a synthetic, gut-restricted bile acid analogue that protects against disease in preclinical models. This discovery establishes a new therapeutic approach with the potential to interrupt recurrent infection and deliver safer, more targeted care for patients.
Discovery: Decoding how bile acids neutralize C. diff’s key toxin
Standard treatment for C. diff infection relies on antibiotics, which remain essential but often disrupt the gut’s natural microbiome. Recognizing the limitations of this approach, SickKids investigators shifted their focus to TcdB, the toxin responsible for most of the cellular damage and inflammation associated with infection.
Earlier work led by Dr. Roman Melnyk, Senior Scientist & Program Head of Molecular Medicine and Co-Director of the SPARC Drug Discovery Facility at SickKids, revealed that certain bile acids do more than aid digestion – they can also block TcdB activity. Building on this insight, postdoctoral fellow Dr. Sean Miletic investigated how these bile acids interact with TcdB inside the gut. In collaboration with Dr. John Rubinstein, Senior Scientist in the Molecular Medicine program and expert in advanced cryo-electron microscopy, the team resolved the first detailed structural view of TcdB bound to bile acids.
These high-resolution images revealed a clear mechanism: TcdB must be in an “open” structural formation to cause cellular damage and specific bile acids can bind to the toxin and hold it in a closed, inactive state.
“It’s like jamming a door at the hinge; if the toxin can’t open into its active form, then it can’t harm cells.”
This structural understanding provided a blueprint for creating synthetic molecules designed to neutralize the toxin directly in the gut.
Translation: Turning the structural discovery into a therapeutic opportunity
This work initiated in the Melnyk lab provided a new therapeutic direction: rather than indiscriminately targeting bacteria, potential treatments could instead disable the toxin C.diff uses to cause disease.
To transform their discovery into a viable therapeutic strategy, the team partnered with collaborators across North America. Leveraging the structural data generated at SickKids, medicinal chemist Dr. Peter Dosa from the University of Minnesota helped design new bile acid analogues optimized to remain within the intestine to maximize their therapeutic effect (Figure 1). Dr. Casey Theriot at North Carolina State University then evaluated these compounds in preclinical models of C. diff infection.
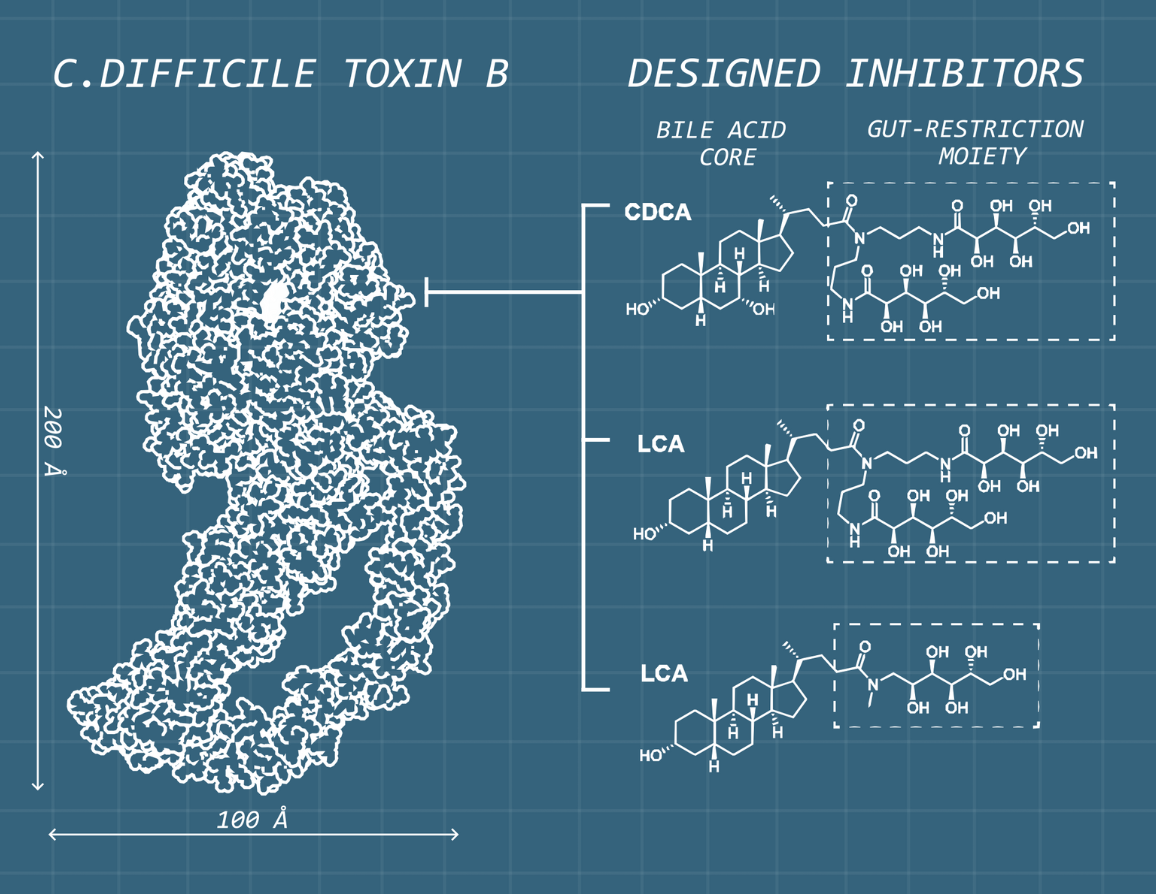
Figure 1.
Using the detailed structure of TcdB and its interactions with bile acids as a blueprint, the research team designed synthetic bile acids with a gut-restriction moiety that allows them to remain in the intestine longer. This targeted design helps the compounds act directly where the toxin causes damage, strengthening their therapeutic potential.
This collaboration resulted in sBA-2, a synthetic bile acid derivative engineered to neutralize TcdB directly. In pre-clinical models, sBA-2 significantly reduced clinical symptoms of disease such as weight loss and intestinal injury. Importantly, it does so without disrupting the gut’s natural microbiome, highlighting its precision and potential as a safer therapeutic approach.
“We are very excited about the potential of sBA-2 as a first-in-class oral therapeutic for this devastating disease. For patients, this approach could mean safer treatment and a real chance to break the cycle of repeated illness.”
Commercialization: Advancing sBA-2 towards clinical development
With structural validation, a clear mechanism of action, therapeutic design, and preclinical efficacy established, the sBA-2 program has reached a critical inflection point. SickKids IP&C has invested strategically through the POP, and now the SickKids Technology Advancement Program (TAP) to de-risk the opportunity and position it for successful translational advancement.
The next stage of development will focus on medicinal chemistry optimization and analogue refinement, pharmacokinetic and toxicology evaluation, assessment of formulation and oral delivery, and establishing a potential path toward IND-enabling studies and early clinical development.
Explore the Opportunity!
SickKids IP&C is actively seeking partners to advance this promising therapeutic program.
To access detailed information on the technology, development status, and partnership opportunities, check out the full technology brief available in the SickKids Technology Portfolio.

